“Gypsum” and “Gypsum Board”
“Gypsum” and “Cement”
What is “gypsum”? Remember when you were in school, about chalk in the classroom and white lines on the school grounds.
"Gypsum" is originally a result of the solidification of a mix of "calcined gypsum" and water.
There is a thing called "cement", but in terms of solidifying when it reacts with water, it has the same characteristics as "gypsum (calcined gypsum)". As for applications of solidified forms of these materials in our daily lives, cement is used as mortar and concrete, and gypsum (calcined gypsum) is used as a gypsum board.
Both are indispensable materials for building construction, but the process of their production and the nature of the products generated are quite different.
Gypsum (calcined gypsum) will harden in minutes to tens of minutes when it reacts with water, so mass production is possible! Dozens are produced each minute in the factory! (Usually, hardening of cement takes several hours to several days.)
Gypsum Board production flowGypsum Boards are recycleable. Also, the process of raw material production does not harm the environment!
On the contrary, it also helps prevent pollution.
It does not shrink during the hardening process. Therefore,cracking does not occur!
Chemical reaction of gypsum

 Gypsum in its base, stone form
Gypsum in its base, stone form
The "gypsum" we see is calcium sulphate with two molecules of crystallized water, usually called "gypsum dihydrate".
- When gypsum dihydrate is heated to 120 °C ~ 150 °C, it will lose 3/2 of the total crystallized water and become "calcined gypsum".
- When water is added to "calcined gypsum", a hydration reaction occurs, and then it returns to the original "gypsum dihydrate" and solidifies.
Utilizing this property, a gypsum board is made by pouring water to kneaded "calcined gypsum" between two sheets of thick paper (liner) and solidifying it into a plate shape.
Adhesion between liner and gypsum
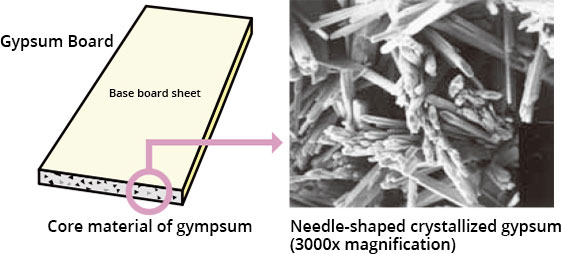
Gypsum board is made mainly of paper(generally called as liner) and gypsum. When you convert "calcined gypsum" and water into "gypsum dihidrate", needle-shaped crystals are formed and the gypsum solidifies. The bonding of paper and gypsum uses the mechanism that this needle-like crystal digests into the fiber of the paper and integrates it. To begin with, it is not made through the method of solidifying gypsum, turning it into a board, and pasting paper on it with glue.
Why Gypsum is Fire Resistant
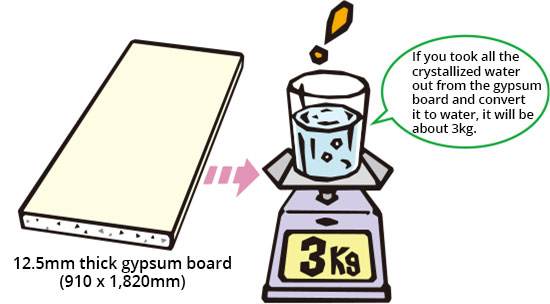
Gypsum is strong against fire. The secret is in crystallized water equivalent to about 21% of its weight.
This crystallized water is very stable in normal condition and does not disperse, but once it comes in contact with the heat of fire it will cause thermal decomposition and start to evaporate. The temperature of gypsum does not rise above a certain temperature until all of the crystallized water evaporates into water vapor and is released. This is the same phenomenon that occurs when you blow a flame with a burner onto a lump of ice, that part gradually dissolves into water and the ice temperature is kept below 0°C until all the ice melts.
Fire resistance